In this paper, the authors continue their investigation and quantitative assessment of how much iodine is needed for optimal health and function of the whole body. As the title emphasises, the primary aim in this paper is to determine the ideal amount of supplemental iodine in light of the fact that it is difficult to get as much as is needed through diet alone in most of the world.
The major themes are 1) what the authors have termed iodophobia, the widespread but entirely unfounded fear of iodine, which continues to pervade in the mentality of physicians, and consequently, in that of the general population; 2) iodine needed for optimal thyroid health; and 3) iodine needed for extra thyroidal tissues. Because the effects of prolonged iodine deficiency in cells leads to cancer, and because the second-most iodine-dependent tissues are those of the mammary glands of the breasts of females, much of the discussion is concerned with cancer of the thyroid and breasts.
The conclusion is the same as in their first paper: an optimal daily amount of iodine is around 12.5 mg of which 5 mg is in the form of iodine, primarily for the thyroid gland, and 7.5 mg in the form of iodide, primarily for the breasts and other extra-thyroidal tissues.
Orthoiodosupplementation: Iodine sufficiency of the whole human body by Guy E. Abraham, MD, Jorge D. Flechas, MD, and John C. Hakala, RPh

Supplemental iodine for optimal health of thyroid, breasts, skin, and whole body. Long-term average daily iodine intake according to authors should be at least 12.5 mg, and provide both iodine and iodide.
The paper begins with the authors’ motivations, presented clearly in the first two paragraphs. They contrast, on the one hand, that it is known and recognised that iodine is the only element required for and in the synthesis of hormones; that these hormones are involved in embryogenesis, differentiation, cognitive development, growth, metabolism, and regulation of body temperature; that iodine is most concentrated in the thyroid; that iodine is the most deficient trace element in the world with more than one third of the world’s population known to be clearly iodine-deficient; and that low iodine is the world’s leading cause of intellectual deficiency.
Whereas, on the other hand, that optimal amounts of iodine for the human body have never been evaluated nor determined; that supplementation has been considered adequate if it prevented cretinism, simple goitre, and symptoms of hypothyroidism; that it has been assumed that the role of iodine was essentially restricted to the synthesis of T3 and T4, so much so that it has become dogma; and that when thyroid stimulating hormone (TSH) assays became available, iodine urine testing was abandoned as irrelevant, and eventually forgotten to the point where today, most clinical doctors will go through the entire career without ever ordering a urine iodine test.
Iodophobia
The fear of iodine, which most likely has its roots in the work of Wolff and Chaikoff (1948), and which we will examine on another occasion, is present and widespread in the literature for all audiences. It is found in the textbooks used in medical schools, in professional journals, in non-technical publications that appear in health magazines, and in books written for the general public by medical professionals. The authors present a number of examples from different sources spanning this range of different kinds of publications intended for different audiences.
They seem to attribute much of the burden for the spread of iodophobia in the US, at least in the last few decades, to one individual, an endocrinologist by the name of Ridha Arem, who was a longtime editor of a professional periodical read by at least 25k endocrinologists throughout the country, and the author of the popular book The thyroid solution: A revolutionary Mind-Body program that will help you, first published in 1999 and currently in its third edition (2017).
In this book, on page 305 of the 1999 edition, Arem writes: “research has clearly established that the high dietary intake iodine content in some areas of the world has resulted in a rise in the prevalence of thyroiditis and thyroid cancer.” A single reference is given in support of this statement: a paper written by Harach & Williams, entitled Thyroid cancer and thyroiditis in the goitre region of Salta Argentina, before and after iodine prophylaxis, and published in 1995 in the journal of Clinical Endocrinology (43:701-6). In this paper, however, no high iodine intake is present or involved in any part of the study.
Harach & Williams (1995) measured urine iodine before and after introduction of iodised salt, and evaluated thyroiditis and thyroid cancer rates. Urine iodine was 9.3 +/- 1.7 mcg/g creatinine before and 110 +/- 13 mcg/g of creatinine after iodisation. There was no change at all in the rates of invasive forms of cancer, and for papillary carcinoma the numbers were 0.78/100k/year before and then 0.84/100k/year after iodisation, which they recognise as insignificant. Not only do these data not support Arem’s claims, but they are not even applicable to an evaluation of the potential effects of high iodine intake. Arem does not provide any other references.
On the same page Arem also writes: “to function normally, the thyroid requires 150 mcg/d … In the US, iodine consumption ranges between 300-700 mcg/d.” No reference is given to support this statement. And this statement is demonstrably false: the National Health and Nutrition Examination Survey, NHANES III (1988-1994), showed that the median iodine in urine was 145 mcg/L, and that at least 15% of US women were markedly deficient, with less than 50 mcg of iodine per litre of urine.
The reason why measuring iodine in urine over 24 hours is a good way to evaluate iodine sufficiency, is because most of it is excreted. If the body’s tissues (thyroid glands, breasts, stomach lining, skin, etc) have all the iodine they need, then we would excrete close to the entirety of the iodine we consume. The greater the discrepancy between ingested and excreted iodine, the greater the deficiency. But because it is water soluble and hard to store, a long time is needed to replenish iodine stores in the tissues. Hence, for this reason, supplementation with larger doses than those needed for optimal maintenance, and extended over many years, are usually needed to restore iodine sufficiency and balance within the body’s most iodine-dependent tissues like the thyroid, breasts, and skin.
In a review paper on iodine ‘excess’ published in 2000 and included in a reference textbook used by endocrinologists in a section entitled Iodine as a pathogen, Roti & Vegenakis the authors report the decline in iodine intake in the US, stating that in 1971-74, it was found that 27.8% of people tested excreted more than 500 mcg/L, whereas in the intervening 15-20 years, this number dropped to 5.3% (1988-1994). Having taken—entirely arbitrarily—500 mcg/L as indicative of excess iodine, the authors present these figures as encouraging and positive in the prevention of iodine ‘excess’, completely ignoring the remarkable discrepancy with the observations of mainland Japanese that show both an iodine intake that is 100 times greater than the US average, and the lowest incidence of goitre and hypothyroidism: figures presented by Finley & Bogardus in 1960, and more recently also in further studies by Thomas et al. in 1983 and 1986.
Moreover, in their review, Roti & Vegenakis note that Amiodarone, a drug commonly used to treat heart arrhythmia, contains 75 mg of iodine per 200 mg tablet (note that this is mg and not mcg), and causes hypothyroidism in 25% of patients that take it. They automatically attributed this to the iodine, but do not investigate the issue further, either by looking at studies on high iodine intakes, or by themselves organising a trial to test this hypothesis, treating arrhythmia using iodine alone without the other pharmaceuticals found in Amiodarone. No such trial has ever been carried out, by the way. Only comparisons between different pharmaceutical drugs.
As a third example of iodophobia and misinformation about iodine in the US, the authors use Dr Shames’s article in the July 2002 issue of Bottom Line Health magazine, and debunk three statements of fundamental significance:
1) Shames writes that iodine deficiency is a thing of the past. However, as mentioned above, NHANES III (1988-1994) found 15% of women to be iodine deficient.
2) Shames writes that iodised salt is sufficient to prevent iodine deficiency. However, iodised salt contains at most 75 mcg of iodine per gram, and since most people eat around 5 g/d, this makes at most 375 mcg/d. This amount may be enough to prevent cretinism and goitre, but to obtain even the bare minimum of 5 mg needed by the thyroid, one would have to eat 65 g of iodised salt per day, which is obviously absurd.
3) Shames writes that people living near coastlines could even be getting too much iodine. However, studies in several countries found no difference in iodine intake between inland and coastal regions.
The unfortunate reality is that all those people who will have read either Arem’s books, Roti & Vegenakis’s reviews, Shames’s articles, or any other published works expressing in similar terms, from a position of authority, statements unsupported by evidence or simply and demonstrably false, will rarely be in a position to question or doubt their validity, and will therefore be left with the entirely unfounded negative predisposition towards iodine transmitted by the authors of these publications.
Iodine for the thyroid gland
The cold war was a period during which the fear of nuclear war, and the subsequent nuclear fallout that would sweep across the region around the explosion was very strong. This fear was shared by most people: parents and grandparents, political leaders and scientists. It was known that the thyroid concentrates iodine: more than 100 times the concentration of other organs and tissues (modern measurements in Delange 2000). It was also known that nuclear fallout would come with a release of large amounts of radioactive iodine in the environment. The nuclear explosion was therefore, in its immediate aftermath, most dangerous for its devastating effects on the thyroid: the thyroid gland would soak up all of that radioiodine, which would destroy it, breaking down its cells from within.
The only way to prevent the thyroid from soaking up all that radioactive iodine from the nuclear fallout would be to fill up the receptors of its cells with normal iodine, and thereby minimise the capture of the radioactive isotopes from the explosion. Because iodine is water soluble and not stored very well, to both provide the thyroid with the iodine it needs and protect it from radioactive iodine in the case of a nuclear accident, one would have to take moderately high amounts of iodine every day, or a very large amount as soon as possible before, during or after exposure, and continue for the early period following the explosion, until the levels of radio iodine contamination have dropped. The iodine receptors in the thyroid, breasts, and other tissues being occupied by normal iodine, the radioactive isotopes would have nowhere to latch on, and would therefore simply be excreted in the urine.
Studies were carried out to determine the amount needed to suppress uptake of radioactive iodine. A defined amount of supplemental iodine would be taken, and then a fixed amount of radioiodine administered. Measuring the amount of radioiodine retained by the thyroid in proportion to the amount administered would give the protection factor associated with the amount of supplemental iodine.
Several groups did such experiments. The results of five groups are presented in Figure 1 below. On the x-axis, we have the amount of iodine in mg consumed per day. On the y-axis, we have the percentage of radioiodine taken up by the thyroid. Naturally, the less iodine is consumed, the higher the percentage of radioiodine retained by the thyroid, and conversely, the more supplemental iodine is taken, the lower the percentage of radioiodine uptake.
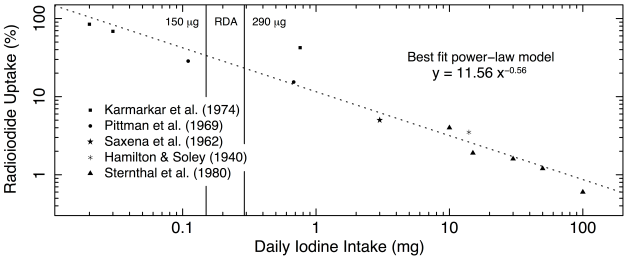
Figure 1. Percentage of radioactive iodine absorption as a function of daily iodine intake. The data are those presented in Table 1 and Figure 1, and the original papers from which they were taken are listed and represented with different symbol. The RDA range is shown by vertical lines at its lower and upper limits of 150 and 290 mcg. The data are presented on a log-log scale. The best fit power-law model is shown as the dashed line, and its parameter values are given.
The greatest protection is conferred by the highest amounts of supplemental iodine, as we can see on the right end of the scale: taking 100 mg/d results in a mere 0.5% uptake, and implies excretion of 99.5% of the radioactive isotope of iodine. At 50 mg/d, uptake is around 1.5%, and excretion around 98.5%. At 20 mg/d, uptake is still below 2% with excretion over 98%, and even at 3 mg/d, uptake is only around 5%, with 95% excretion of the radioactive iodine.
The scale, both on the x-axis and on the y-axis, is logarithmic. This means they have equal spacing in powers of 10. And so, the tick marks between 0.1 and 1 represent steps of 0.1, those between 1 and 10, represent steps of 1, and those between 10 and 100 represent steps of 10 units. The units are mg on the x-axis, and percentage points on the y-axis. A linear relationship (a straight line) in log-log space, as the one we see in this plot, shows to a power-law relation, and power-laws tell us that change is very fast.
In this case, this tells us that increasing iodine intake from nothing to even a little bit, makes a big difference in terms of decreasing the uptake of the radioactive iodine. On the other hand, it also means that as we keep increasing the amount of supplemental iodine, the decrease in uptake becomes less and less significant. Hence, it is very easy to protect the thyroid against nuclear fallout by decreasing uptake of radioiodine from 100% to 20% by taking just 0.7 mg of iodine per day, but to get maximum protection, we need to take 50–100 mg/d. The great news is that we can get full protection, without having to worry about a thing from all this supplemental iodine, because it is basically harmless, and excesses are eliminated.
The authors present these data (together with other data that we don’t discuss here) in a table, and then in a graph, which is logarithmic only in x, but linear in y. Therefore, they interpret the relation—which is clearly linear in log-log space, but not in semi-log space—as showing evidence of four different parts with different slopes and different physiological meanings. I believe the single power-law is both simpler and more natural a model to characterise the relationship between supplemental iodine and radioactive iodine uptake by the thyroid. I therefore skip reporting on the details of their analysis of the slopes and x-axis intercepts and interpretation of their meaning.
In addition, the authors rightly point out that none of these studies were intended to measure the optimal amount of supplemental iodine. They were motivated by providing a framework for crisis management in the event of a nuclear war. Nevertheless, their scientific value in understanding iodine needs for optimal thyroid function is indeed great. Other studies intended on measuring thyroid absorption of iodine are mentioned: those of Thompson et al (1930), Wagner et al. (1961), and Fisher et al (1965), all pointing to a maximal absorption rate of iodine of about 600 mcg/d. This is interesting, but not enough because absorption rate will depend on state of deficiency or sufficiency, and will also evolve as iodine levels are replenished, assuming more iodine is provided than is absorbed. But two other cases stand out.
Plummer, a clinician who treated people suffering from Grave’s disease, a severe form of goitre, hypothesised that the hyperthyroidism associated with this condition was caused by iodine deficiency, and furthermore, that it was this deficiency that also caused such a high post-operative mortality rate. He therefore gave his patients 20-30 drops of Lugol’s solution before and 10 drops after operations—that’s 125-187.5 mg before and 62.5 mg after—and happily saw the mortality rate drop to zero. Of course, this didn’t prove his hypothesis as correct; this is never really possible in science. But it is strong supporting evidence, and did show that it was highly likely to be the case. And given that he knew iodine supplementation was harmless, he also knew that it could only help. He was right, and the benefit to his patients couldn’t have been greater: it was life over death. It was, naturally, an easy decision to make. He knew that, and now, so do we.
Koustras et al (1964) performed extensive studies with meticulous accounting of iodine balance on people to quantify the relationship between the amount ingested and retained over a period of several weeks with daily supplementation. This is what they concluded: “From our evidence, it appears that, from all the doses we used, the thyroid took up about 6-7 mg of iodine before an equilibrium in plasma inorganic iodine was reached.” This seems to be, from several lines of evidence, a good estimate of what the thyroid needs.
Iodine for the mammary glands and other tissues
Having established that the thyroid needs 6-7 mg of iodine per day, the authors need to estimate how much is needed by the rest of the body. Because breast tissue concentrates as much iodine as the thyroid, and because, as reported previously, goitre is six times more—that’s 600% more—common in teenage girls as it is in teenage boys, it is essential to consider iodine needs of the mammary glands. Here are some facts the authors present that are associated with the problem of iodine deficiency in women:
- Japanese have the world’s highest intake of iodine (14 mg/d from 5 g of seaweed, on average), and the lowest incidence of goitre, hypothyroidism, and breast cancer (Finley & Bogardus 1960; Thomas et al 1983, 1986).
- There is a strong inverse correlation between iodine intake and cancers of the breasts and ovaries, and a strong positive correlation between thyroid volume and breast cancer incidence: 13 ml in Irish women without versus 20 ml in women with breast cancer (Thomas et al 1983, 1986).
- There is a strong inverse correlation between free T4 and breast cancer. In 5 different ethic groups from Hawaii, Britain and Japan, the highest levels of free T4 in Japan were associated with the lowest incidence of breast cancer. But T4 therapy doubles incidence of breast cancer. Therefore, it is obviously not T4 that protects against breast cancer in Japanese women, but iodine, which, at the same time, ensures optimal T4 levels. (Ghandrakant, Kapdim & Wolfe 1976; Hinze et al. 1989)
- The amount of iodine needed to prevent FDB and breast cancer is at least 20-40 times greater than what is needed to prevent goitre (Esquin et al. 1995).
- Thyroid and skin concentrates iodide; breast concentrate iodine. Both are needed.
- US intake is about 100 times less than in Japan. In the 1960’s iodine was used as an anti-caking agent in flour, which made the average intake approximately four times greater than it is today. Incidence of breast cancer was then 1 in 20. Iodine in flours was replaced by iodine-displacing bromine. Incidence of breast cancer in 2000 (publication date) reported as 1 in 8.
- Iodine deficiency is without a doubt just as important a cause of thyroid cancer as it is of breast cancer. In 2001 in the US, there were 19500 new cases of thyroid cancers, and of these, 14900 were in women. That’s 75%. Now, in 2017, estimates are for 56870 new cases of which 42,470 will be in women. That’s still 75%, and it’s also about 400% more cases than 15 years ago.
To determine with the greatest precision where iodine is most concentrated within the tissues of the body, and how much is kept, Berson and Yallow (1954) used traceable radioiodine to determine, in addition to what has already been discussed about iodine being most concentrated in the thyroid, breasts, and skin, that the total exchangeable pool of inorganic iodine ranged from 7 to 13 mg across their study. This means, that besides those most iodine-dependent tissues that trap and concentrate it, the body as a whole uses at least this amount on a daily basis.
Given this large amount used by the whole body, iodine must have several other functions besides protecting the structure and ensuring the proper physiology of the thyroid and breasts. Derry (2001) reviewed iodine’s general properties and benefits to a healthy body. He found that iodine works in organs as an antimicrobial agent, that it has a potent apoptotic function in the body’s surveillance mechanisms against abnormal cells, that it has the ability to trigger differentiation, and that, in addition, iodine has powerful antioxidant properties, which confer it equally powerful protective effects on the DNA of cells, because it enhances the singlet to triplet transition, and because the most damaging reactive oxygen species that damage our DNA and other large molecules are usually singlets.
Naturally, these effects strongly depend on the concentration of the available iodine circulating in the fluids of the body. Using fluid concentration measurements in the work of Szent-Gyorgy (1957), the authors estimate that an average daily intake of 12.5 mg of iodine, which at the same time, they underline, would offer protection from nuclear fallout at the 3-4% level, would also be sufficient to confer all of iodine’s antimicrobial, apoptotic, antioxidant and DNA-protecting effects.
Epilogue
The paper ends with an epilogue where the authors express some difficulties in understanding, in the context of evolution, why humans would have evolved needing so much iodine while recognising how hard it is to obtain as much as is needed. In my opinion, there is no difficulty there from the perspective of evolutionary theory. The first homo sapiens in our lineage, those that developed speech, swept across the world, and came to dominate every last part the planet, in all likelihood evolved on a coastline somewhere in south western Africa eating seafood and seaweed. Many believe that it was their diet, rich in animal foods from the sea that gave them this advantage over other species and even other sub-groups of sapiens scattered here and there on the continent. In fact, it is very likely that it was their iodine-rich diet that conferred to them this evolutionary advantage, which was the intelligence for which sapiens are known.
For most of our evolutionary history, bands of humans would have continued to live near coastlines because of all the obvious advantages this offered. As local and global populations grew, bands would scatter in search of more readily available resources and less competition in their ability to access and use them. Those groups that stayed on the coastlines or in areas where the soil was rich in iodine, became the most successful because they were the most intelligent. Those groups that went further inland or lived in areas where the soil was poor in iodine, grew progressively less intelligent and less successful from one generation to the next.
There is no problem at all with such a scenario, and, in fact, modern observations and data collecting techniques confirm this: areas where iodine deficiency is common, have the highest incidence of hypothyroidism, goitre, breast cancer, thyroid cancer, but also cretinisms and intellectual deficiency. As attested by a joke used in some towns in the goitre belt when someone does or says something stupid: “Are you iodine deficient, or something?”
It wouldn’t at all be surprising if, with sufficiently large data sets, we found a strong and tight correlation between iodine intake and IQ levels within populations from the same genetic pool, but also globally across diverse populations from different gene pools. Many other factors come into play. Nevertheless, iodine during pregnancy and childhood is certainly one of the most important for proper intellectual development.
For us, each with our own particular genetic makeup and recent ancestral evolutionary history, each with our personal and family history, each with our time in our mother’s womb, our childhood and teenage hood upbringing and diet, what this means is that we better make sure we take all the iodine we need to first correct, and then prevent the wide spectrum of problems that iodine deficiency and iodine insufficiency bring about. Might as well maximise our health as well as intellectual potential in this simple way. The costs are insignificant, the risks quasi non-existent, and the potential benefits are tremendous.
Summary
This paper is very similar in spirit and purpose to the authors’ first paper. In this second paper, they recall and restate several points they had made in the previous, and extend their detailed investigation of how much iodine is needed for optimal health and function of the whole human body. The main points to remember are that:
- The fear of iodine is widespread, but wholly unjustified and unfounded.
- Iodine is most highly concentrated in the thyroid gland.
- Iodine is essential and crucial for the normal development, and subsequently, normal function of the brain through its action on the thyroid gland.
- Iodine deficiency is the world’s leading cause of intellectual deficiency.
- In females, iodine is equally concentrated in the breasts as it is in the thyroid.
- Iodine deficiency is known to cause cretinism and intellectual deficiency, hypothyroidism and goitre, nervousness, anxiety and restless leg syndrome, fibrocystic breast disease, thyroid cancer, and breast cancer.
- Iodine deficiency causes goitre in women 6 times more often than in men.
- Breast cancer now affects 1/8 women. In the 1960’s it affected 1/20.
- Thyroid cancer rates have quadrupled in 15 years from 2001 to 2016.
- 75% of thyroid cancer cases are in women.
- The thyroid gland needs approximately 6 mg of iodine per day.
- The mammary glands and rest of the body need approximately 6-8 mg per day.
- The Japanese are the only known population with iodine sufficiency from diet, which provides on average 14 mg of iodine from seaweed.
- Minimum average requirement for iodine sufficiency is around 12.5 mg/day.
- As is the case for most micronutrients, some people need more, some less.
- It will often be necessary to consume a lot more for extended periods in order to overcome and/or reverse the effects of a long-standing insufficiency or deficiency.
- Maximum protection of the thyroid from nuclear fallout is gotten at 50-100 mg/day.
We will continue this series with an article by the same three authors entitled: Measurement of urinary Iodine Levels by Ion-Selective Electrode: Improved Sensitivity and Specificity by Chromatography on Anion-Exchange Resin.
If you think this article could be useful to others, please Like and Share it.
Just wonder.. I supplement with Lugols drops (20% strength) dermally. What is your opinion about those – are they absorbed and used by the body correctly?
LikeLike
Yes
LikeLike
How can I get in contact with you? I can’t find a way to do so. Thank you for your work.
Sašo
LikeLike
It depends about what, but this is a good way: https://www.patreon.com/healthfully
LikeLike
Pingback: I’m trying iodine. – Fitter at Fortyish
If a person is taking 75 mg of T3 is there a risk of too much iodine if one supplements with iodine too? Thank you.
LikeLike
I would say yes. You should substitute the T3 for iodine. But you should do it very gradually.
LikeLike
I am self treating Hashimotos and only feel well on 75 mcg T3. Do you believe a person can self treat Hashimotos with iodine. Thank you.
LikeLike
Yes, I’ve read it’s doable. Try it by shifting from less T3 to more Iodoral.
LikeLike
Dear Mary, would you mind telling us if you have had success in increasing iodine and decreasing T3?
LikeLike
That isn’t good advice at all, that “you should substitute T3 for iodine”. It doesn’t work like that…It’s true that some people will be able to lower their meds and in some cases, if the immune system hasn’t done too much damage yet, you may even be able to go off meds. But Hashimoto’s destroys your thyroid cells which means you have less thyroid tissue left and so are unable to produce adequate amounts of thyroid hormone. Iodine will not regrow your thyroid tissue back in fact nothing will (they’re starting research on cold laser therapy but it’s in its infancy).
Just take it easy and if you go hyper (as per tests) then lower your T3 bit by bit. If enough of your gland is missing already then, even if you manage to put Hashi’s into remission, you will always need an external source of hormones. Iodine is not a substitute for thyroid hormones.
LikeLike
Thanks for the comment. Iodine-savvy MDs do that. But I suppose it shouldn’t be generalized without a careful assessment. Nevertheless, there’s no harm in trying gently to move away from thyroid supplements towards more iodine since it has been shown to be possible in some cases.
LikeLike
What is a better test blood serum iodine or urine iodine
LikeLike
The test for iodine is 24-hour urine test. You take 50 or 100 mg in the morning, then collect all of your urine for 24 hours, and the lab measures how much iodine there is in the urine. The level of deficiency is inversely proportional to the amount in the urine: the more deficient you are, the less there is in the urine; when most of the iodine taken is in the urine, it means your stores are good. So, you need to find a place where they do a 24-hour iodine sufficiency test.
LikeLike
Hi
I am on 5mg carbimazole for my hyperthyroid, would iodine be a good choice if I stop the meds or take the iodine with the meds and slowly taper the meds down
LikeLike
Hi Amal, I’m not in a position to give personal medical advice. The only thing I can reiterate is that iodine supplementation is essential for healthy thyroid function, and that deficiencies can lead to both hypo and hyper thyroid that can be corrected with supplementation. Transition off meds should always be done gradually.
LikeLike